It has been over a year of pandemic living. Many of us are tired of all the restrictions and are looking forward to a return to business as usual. With the roll out of vaccines from up to four different pharmaceutical companies, it is our hope that the end may be in sight.
To my great surprise and pleasure, the Nigerian government has procured vaccines for the general public and they are being administered for FREE at many Primary Health Care centres around the country.
Understandably, some people are skeptical about taking the vaccine as a result of conspiracy theories being peddled in Whatsapp groups. As a health care professional who has also had her first dose of the AstraZeneca COVID-19 Vaccine (which is the brand currently available in Nigeria), I hope to dispel some of these theories and hopefully educate, reassure and convince you to take the vaccine.
About the vaccine
COVID-19 Vaccine AstraZeneca is indicated for active immunisation of individuals ≥18 years old for the prevention of coronavirus disease 2019 (COVID-19).
The COVID-19 Vaccine AstraZeneca vaccination course consists of two separate doses of 0.5 ml each. The second dose should be administered between 4 and 12 weeks after the first dose
It is recommended that individuals who receive a first dose of COVID-19 Vaccine AstraZeneca
complete the vaccination course with COVID-19 Vaccine AstraZeneca.

How safe is the COVID-19 vaccine?
The overall safety of COVID-19 Vaccine AstraZeneca is based on data from four clinical trials conducted in the United Kingdom, Brazil, and South Africa. Majority of the study participants were aged 18yrs and above, therefore the safety and efficacy of COVID-19 Vaccine AstraZeneca in children and adolescents (aged <18 years old) have not yet been established.
Any coronavirus vaccine that is approved must go through all the clinical trials and safety checks all other licensed medicines go through.
The vaccines approved for use by the public have met strict standards of safety, quality and effectiveness set out by the appropriate regulatory bodies which follow international standards of safety.
Duration and level of protection
The duration of protection has not yet been established.
As with any vaccine, vaccination with COVID-19 Vaccine AstraZeneca may not protect all vaccinated individuals. This should not discourage anyone from taking the vaccine because the data from the study has shown more people achieving immunity than those who did not.
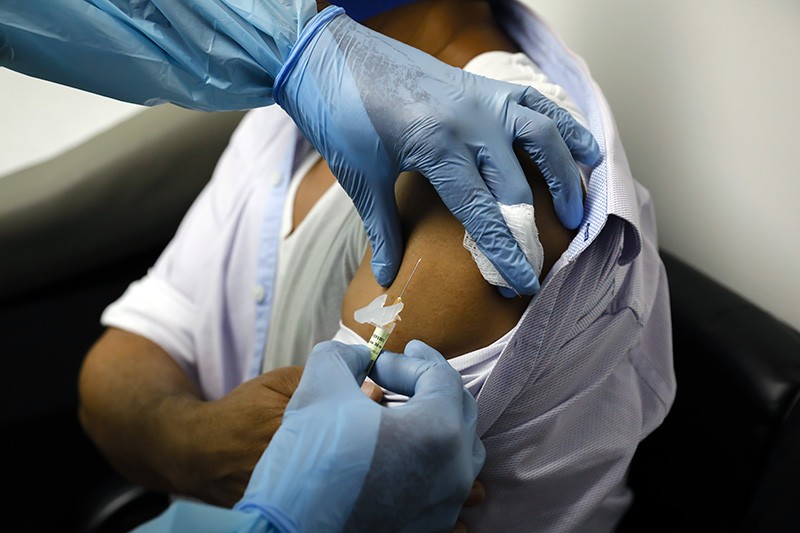
How the COVID-19 vaccine is given
The COVID-19 vaccine is given as an injection into your upper arm.
It is given as 2 doses. You will have the 2nd dose 12 weeks after having the 1st dose.
Who should be given priority
Priority should be given to:
● people aged 50 and over
● people who live or work in care homes
● health and social care workers
● people with a condition that puts them at higher risk (clinically vulnerable people with pre existing medical conditions such as asthma, hypertension, diabetes, sickle cell and so on)
● people with a learning disability
● people who are a main carer for someone at high risk from coronavirus
Advice if you’re of childbearing age, pregnant or breastfeeding
There’s no evidence the COVID-19 vaccine is unsafe if you’re pregnant. But more evidence is needed before it can be routinely offered to pregnant women.
However, you may be able to have the vaccine if you’re pregnant and:
● at high risk of getting coronavirus because of where you work
● have a health condition that means you’re at high risk of serious complications of coronavirus
Administration of COVID-19 Vaccine AstraZeneca in pregnancy should only be considered when the potential benefits outweigh any potential risks for the mother and fetus.
You can have the COVID-19 vaccine if you’re breastfeeding.
You do not need to avoid pregnancy after vaccination. The vaccine cannot give you or your baby COVID-19.
Concurrent illness
As with other vaccines, administration of COVID-19 Vaccine AstraZeneca should be postponed in individuals suffering from an acute severe febrile illness. However, the presence of a minor infection, such as cold, and/or low-grade fever should not delay vaccination.
Side effects
So far, millions of people have been given a COVID-19 vaccine and reports of serious side effects, such as allergic reactions, have been very rare. No long-term complications have been reported so far.
There have been reports in some countries of a small number of people having blood clots after the Oxford/AstraZeneca COVID-19 vaccine.
The Medicines and Healthcare products Regulatory Agency (MHRA) says the current evidence does not suggest the clots were caused by the vaccine and you should still get vaccinated when you get the opportunity.
I have attached an infographic to show the risk of blood clots associated with other medicines/conditions.
The most common side effects according to the trial are:
● injection site tenderness (63.7%),
● injection site pain (54.2%),
● headache (52.6%),
● fatigue (53.1%),
● Muscle aches (44.0%),
● malaise (44.2%),
● High body temperature (includes feverishness (33.6%) and fever ≥38°C (7.9%)), chills (31.9%),
● joint aches/pain (26.4%)
● and nausea (21.9%).
If you have ever taken your child to get immunized, you may recognize some of these as fairly common side effects that can occur with any vaccine.

The majority of adverse reactions were mild to moderate in severity and usually resolved
within a few days of vaccination.
If required, analgesic and/or anti-pyretic medicinal products (e.g. paracetamol-containing products) may be used to provide symptomatic relief from post-vaccination adverse reactions.
Speaking for myself, I had absolutely no side effects afterwards and completely forgot I had even received my vaccination. However some recipients have reported chills, shivering (in some cases rigors), and increased body temperature possibly with sweating, headache (including migraine-like headaches), nausea, myalgia and malaise, starting within a day of vaccination. These effects usually last for a day or two.
If a patient reports unusually high or prolonged fever, or other symptoms, alternative causes should be considered. Contact your doctor if you have a headache for more than 4 days after your vaccination or get bruising somewhere other than where you had your vaccination.
Allergic reactions
Tell healthcare staff before you are vaccinated if you’ve ever had a serious allergic reaction.
You should not have the COVID-19 vaccine if you have ever had a serious allergic reaction (including anaphylaxis) to:
● a previous dose of the same vaccine
● any of the ingredients in the vaccine
Serious allergic reactions are rare. If you do have a reaction to the vaccine, it usually happens in minutes. Staff giving the vaccine are trained to deal with allergic reactions and treat them immediately.
It is my hope that after reading this that you make arrangements to receive your vaccine. If you have any concerns at all, speak to a healthcare professional before you have the vaccination. They will discuss the benefits and risks with you.